French patent filings 2017
June 28, 2018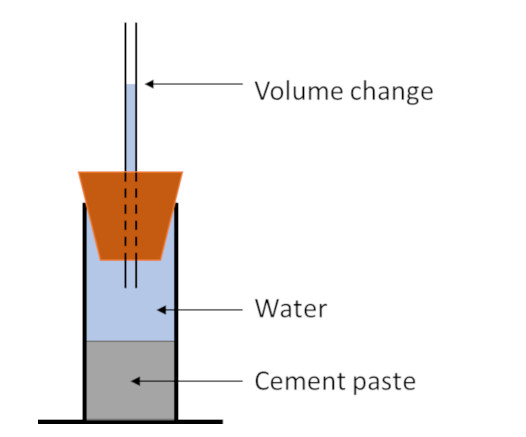
Chemical shrinkage of hydraulic binders
September 4, 2018Shrinkage and expansion of Portland cement systems
The chemical shrinkage of hydrating Portland cement systems, the resulting bulk volume changes and the potential consequences for hydraulic isolation and well integrity in oil and gas wells will be addressed in a series of articles.
The different articles are:
- Introduction: background to cement chemistry and hydration reactions
- Chemical shrinkage: what it is, how to calculate it and how to measure it?
- Bulk volume changes: how do well conditions affect the bulk volume changes of cement and what are the consequences for zonal isolation?
- Expanding agents: how can they be used to improve zonal isolation?
Cement chemical notation
In cement chemistry a special notation is often used to simplify the description of the cement compounds as many cement compounds can be expressed as a sum of oxides.
The common abbreviations of the oxides are:
| C = CaO (lime) | A = Al2O3 (Alumina) | H = H2O |
| S = SiO2 | F = Fe2O3 | S̄ = SO3 |
Thus, tricalcium silicate, Ca3SiO5 can be written as 3CaO.SiO2 which is simplified as C3S
Portland cement components and their reactions
Portland cement is prepared by grinding Portland cement clinker with one or more forms of calcium sulphate, usually gypsum (CS̄H2). The main oxide compositions of Portland cement are:
- CaO 60 – 70%
- SiO2 18 – 22%
- Al2O3 4 – 6%
- Fe2O3 2 – 4%
Mineral Composition of Classical Portland Cement Clinker
| Oxide Composition | Cement Notation | Common Name | Concentration (wt%) |
| 3CaO.SiO2 | C3S | Alite | 50 – 65 |
| 2CaO.SiO2 | C2S | Belite | 15 – 25 |
| 3CaO.Al2O3 | C3A | Aluminate | 5 – 15 |
| 4CaO.Al2O3.Fe2O3 | C4AF | Ferrite phase | 5 – 12 |
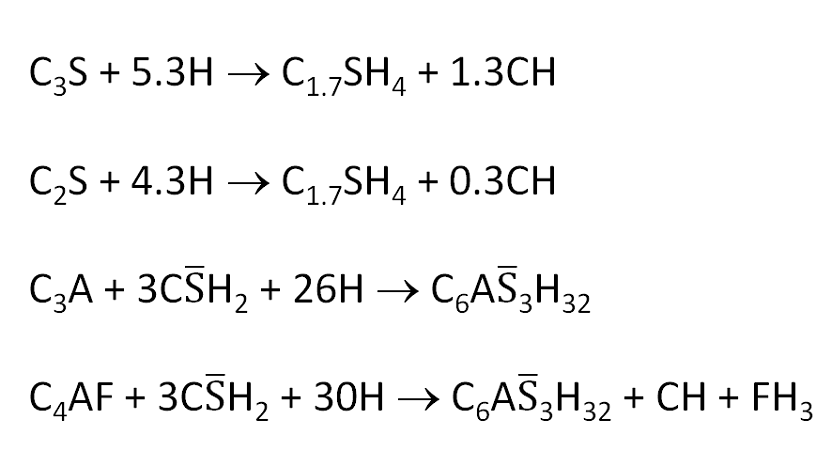
When Portland cement (clinker with gypsum) is mixed with water the hydration of the different components at early times can be written as:
- C3S + 5.3H → C1.7SH4 + 1.3CH
- C2S + 4.3H → C1.7SH4 + 0.3CH
- C3A + 3CS̄H2 + 26H → C6AS̄3H32
- C4AF + 3CS̄H2 + 30H → C6AS̄3H32 +CH + FH3
Equations 1 and 2 represent the hydration of the silicate phases. The composition of the calcium silicate hydrate, written as C1.7S̄H4 in this example, can vary significantly depending on the hydration conditions. CH is Portlandite.
Equations 3 and 4 represent the hydration of the aluminate phases in the case where there is still excess of sulphate ions present, which is more likely at early times. The C6AS̄3H32 is ettringite.