Cement chemistry and hydration reactions
July 17, 2018
Bulk volume changes of Portland cement systems
September 23, 2018Chemical shrinkage during hydration of Portland cement
This is the second article in the series linking the chemical shrinkage of hydrating Portland cement systems to well integrity and zonal isolation in oil and gas wells. the other articles are:
- Introduction: background to cement chemistry and hydration reactions
- Chemical shrinkage: what it is, how to calculate it and how to measure it?
- Bulk volume changes: how do well conditions affect the bulk volume changes of cement and what are the consequences for zonal isolation?
- Expanding agents: how can they be used to improve zonal isolation?
When Portland cement reacts with water, there is an overall reduction in the volume of components:
Volumecement + Volumewater > Volumecement hydrates
This absolute volume decrease is referred to as chemical shrinkage, total chemical contraction or hydration volume reduction.
As an example of how to calculate chemical shrinkage we can take the example of the early hydration of calcium trisilicate (C3S).
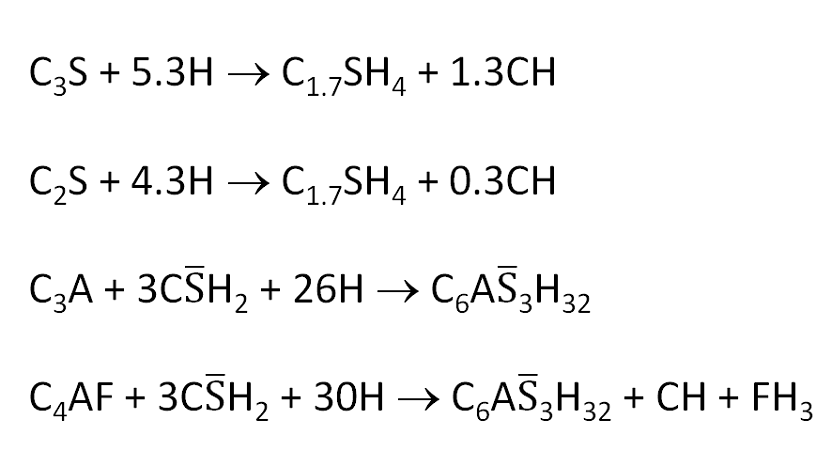
The hydration of C3S at early times is given by:
C3S + 5.3H → C1.7SH4 + 1.3CH………….(1)
To calculate the chemical shrinkage, we need to know the densities of the tricalcium silicate, calcium silicate hydrate and Portlandite. These values are given in the paper by Pierre Mounanga, Abdelhafid Khelidj, Ahmed Loukili, Véronique Baroghel-Bouny, “Predicting Ca(OH)2 content and chemical shrinkage of hydrating cement pastes using analytical approach,” Cement and Concrete Research, Elsevier, 2004, 34 (2), pp.255-265.
From equation 1 above the mass and volume of the reactants and products can be calculated as shown in the table below. For the complete hydration of 1 mole (228g) of tricalcium silicate there is a reduction in volume of 11.9 mL: 5.2mL/100g of C3S
| Density
(g cm-3) |
Molar mass
(g) |
Mass reactants
(g) |
Volume
(cm3) |
|
| C3S | 3.15 | 228 | 228 | 72.4 |
| 5.3H | 1.0 | 18 | 95.4 | 95.4 |
| TOTAL | 323.4 | 167.8 | ||
| Mass products
(g) |
||||
| C1.7SH4 | 2.01 | 227.2 | 227.2 | 113 |
| 1.3CH | 2.24 | 58 | 96.2 | 42.9 |
| TOTAL | 323.4 | 155.9 |
Similar calculations can be performed for the other cement phases as described in the paper cited above.
Chemical shrinkage occurs in other hydrating systems including plaster, lime and calcium aluminate cement and also occurs with the hydration of “expansion additives,” such as magnesium oxide, used in some oil well cementing slurries.
In all these cases chemical shrinkage cannot be avoided, but the negative consequences of the chemical shrinkage can be minimised or eliminated.
Chemical shrinkage during hydration of magnesium oxide
The hydration of magnesium oxide (MgO – periclase) to form magnesium hydroxide (Mg(OH)2 – brucite) is written as:
MgO + H2O → Mg(OH)2………….(2)
| Density
(g cm-3) |
Molar mass
(g) |
Mass reactants (g) | Volume
(cm3) |
|
| MgO | 3.6 | 40 | 40 | 11.1 |
| H2O | 1.0 | 18 | 18 | 18 |
| TOTAL | 58 | 29.1 | ||
| Mass products (g) | ||||
| Mg(OH)2 | 2.24 | 58 | 58 | 24.2 |
| TOTAL | 58 | 24.2 |
Complete hydration of 1 mole of MgO gives a volume reduction of 4.9 mL: 12.3 mL/100g. Expressed in terms of volume reduction per mass of starting material this is twice the chemical shrinkage of C3S, yet MgO is used as an “expanding” agent.
We will see in the next article why this is the case?
Measurement of chemical shrinkage
The principle of the measurement of chemical shrinkage is easy, but experiments must be performed carefully to obtain correct results. See for example ASTM C1608 – 17 “Standard Test Method for Chemical Shrinkage of Hydraulic Cement Paste”.
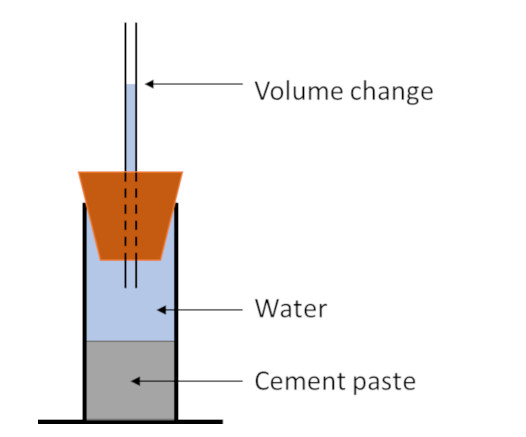
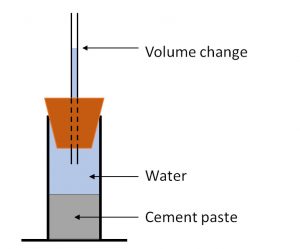
The diagram on the left shows a basic setup to determine chemical shrinkage. A cement paste is mixed, and a known mass placed in a container. The container is closed with a tube with volume graduations and the whole system filled with water. As the cement hydrates the volume reduces and the drop in the water level indicates the volume reduction. This is the dilatometry method.
A variation on this method is to place the whole assembly on a balance and to maintain the water level constant by adding water, the volume of which is indicated by the increase of mass. This is the pycnometry method.
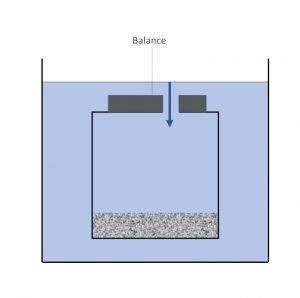
A variation of the pycnometry method is to suspend the entire assembly from a balance in a container of water . In this way the water level is maintained at a constant level and the increase in mass can be monitored continuously from the balance.
In all these methods there are several key parameters that must be controlled to ensure an accurate measurement:
- Temperature: a variation in temperature will create changes in the volume due to thermal expansion or contraction. The test setup must be housed in an environmental chamber to minimise temperature variations.
- Container shape: a wide bottomed vessel such as an Erlenmeyer flask is preferred for several reasons.
- There is a larger surface area available so there is more contact between water and the cement paste.
- There is less restraint of the container walls on the cement paste
- Thickness of the cement paste. To allow penetration of water into the entire cement paste the the cement paste should be between 5 and 10mm thick. If water doesn’t penetrate entirely into the cement paste, then the chemical shrinkage will be underestimated.
- The cement slurry and water must be air free – they should be de-aired prior to addition to the equipment.
- The water to cement ratio (w/c) of the slurry should be optimum – 0.4 is suggested by the ASTM method. If the w/c ratio is too low, it may be difficult for a homogeneous paste to be made and for water to be able to penetrate the entire paste during hydration. If the w/c is too high, then there may be bleeding of the cement paste leading to inhomogeneities.